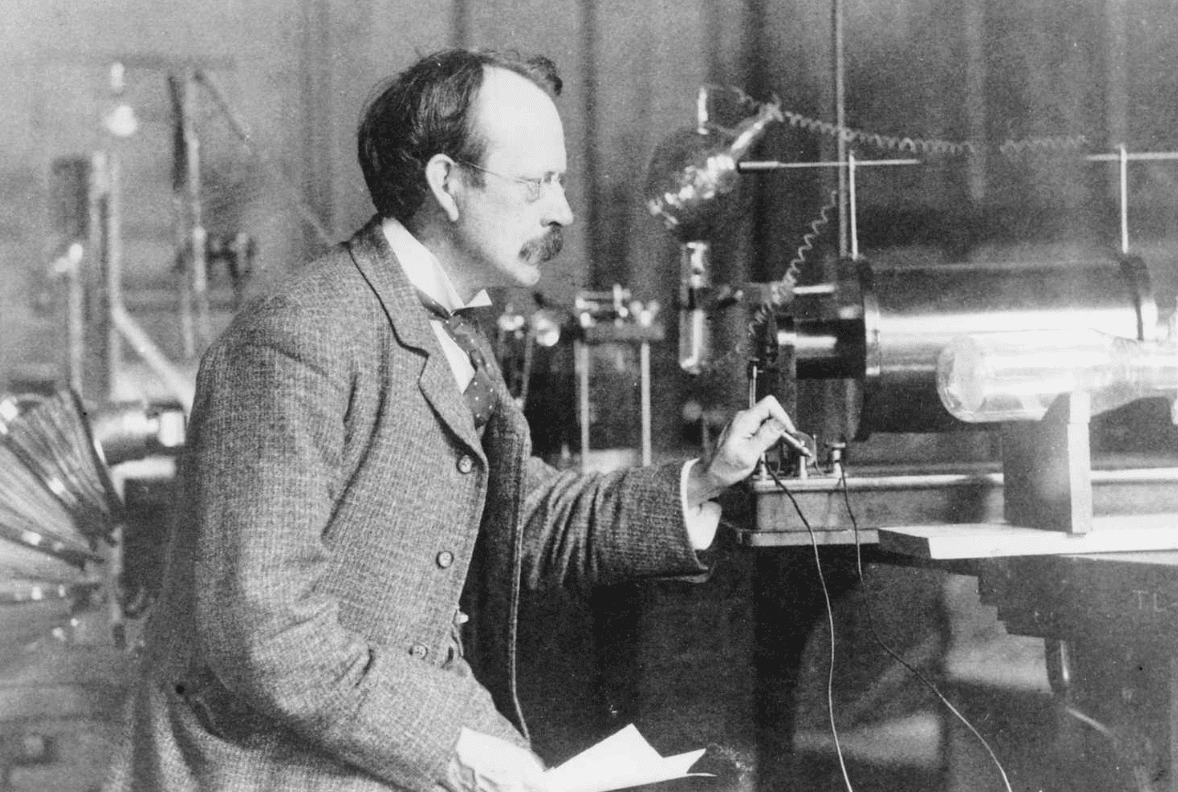Joseph John Thomson was a scientist, physicist and innovator whose name became known not only in Manchester but also throughout the country. Among his many scientific achievements is the discovery of the electron, which stands out as a milestone that has revolutionised our understanding of the atomic structure and the nature of matter. Learn more at manchester.info.
What is known about the physicist?
The future outstanding physicist was born in Cheetham Hill in Manchester in 1856. He grew up in a rather wealthy family. In particular, Thomson’s mother was from a local family of successful textile workers and his father owned an antique bookstore, founded by Thomson’s great-grandfather.
Science absorbed all of young Joseph’s interest and curiosity. Attending local private schools, he was very enthusiastic about learning exact sciences such as mathematics, physics, chemistry and others. Moreover, Thomson was not only very interested in the study, he also showed deep knowledge in the matter.
Hard years of studying at school turned out to be fruitful. In 1870, Thomson was admitted to Owens College in Manchester (now the University of Manchester) at the age of 14. One of the teachers who influenced Thomson, his knowledge and his entire subsequent career was the local famous physics professor Balfour Stewart.
While studying at the university with one of the best professors in the country at such a young age, Thomson began to conduct various physics research as well as experiments with contact electrification. Soon, his first scientific paper was published.
Thomson’s parents were proud of their son’s success, so they wanted to help him with his further career and planned to enrol him as an apprentice engineer at the Manchester locomotive company Sharp, Stewart & Co. However, it was never realised because Thomson’s father died in 1873.
After the death of his father, Thomson decided to delve even deeper into the world of science. After graduating from Owens College, he entered Trinity College, Cambridge. There, he again demonstrated deep knowledge and took an active part in the scientific life of the university. For his achievements, he was awarded a prize twice. At this institution, he earned a bachelor’s degree and a master’s degree in mathematics.
Career ladder
After graduating, Joseph Thomson was appointed Cavendish Professor of Physics at the University of Cambridge. It caused considerable surprise in the scientific community. After all, Thomson was very young, having entered and graduated from university early, with no experience behind. Scientists considered that more mature and experienced candidates, such as Osborne Reynolds or Richard Glazebrook, were more suitable for that position. In particular, they had more experience in laboratory work. Although Joseph Thomson was young at the time, he was widely known for his scientific work as a mathematician. His exceptional talent was known far beyond Manchester.
Thomson devoted his whole life to science and discoveries in physics. During his lifetime, he won a huge number of awards compared to his colleagues. In particular, Thomson was knighted, awarded the Order of Merit and also received the Nobel Prize in Physics.
He gave lectures at the university, wrote scientific papers and remained faithful to his work until the end of his life. But, it is worth noting that perhaps one of Thomson’s most significant discoveries and achievements was the discovery of the electron, the first subatomic particle.
Electron, Thomson’s main discovery

Thomson’s path to the discovery of the electron was quite difficult. It was a time of intense scientific research and debate about the nature of electricity and matter. Thomson’s early work included the study of cathode rays, the streams of particles in discharge tubes. The theory suggested that these rays were a form of radiation, but their exact nature remained unclear.
In 1897, Thomson made a ground-breaking observation while conducting experiments at the Cavendish Laboratory in Cambridge. He noted that cathode rays are deflected by both electric and magnetic fields. This deviation indicated that the rays weren’t just waves, as previously thought, but consisted of particles with mass and charge. By carefully measuring the deviations, Thomson calculated the charge-to-mass ratio of these particles and concluded that they were much smaller than any known atom. His research led to the discovery of a new fundamental particle, the electron.
Revolution in scientific research on atomic theory

The discovery of the electron by the Manchester scientist Joseph Thomson was a monumental leap in scientific understanding and research. Before its discovery, it was believed that the atom was the smallest indivisible unit of matter. The results of Thomson’s scientific research cast doubt on the previous beliefs. Based on Thomson’s research, it was assumed that atoms consist of even smaller particles. This discovery underlies the creation of the modern atomic theory. Moreover, Joseph Thomson enabled further research into the subatomic world.
Having discovered the electron, Thomson proposed an entirely new atom model to the Manchester scientific society. It was also known as the “plum pudding model”. As Thomson saw it, the atom was a positively charged sphere filled with negatively charged electrons, resembling raisins in pudding. This theory was later refined by such British scientists as Ernest Rutherford and Niels Bohr. However, Joseph Thomson became a pioneer in this field, taking a decisive step in the development of understanding of atomic structure.
Still, Thomson’s life wasn’t filled only with discoveries and achievements. He devoted most of his scientific career to lecturing. During his lifetime, he raised a huge generation of scientists who made a significant contribution to physics and also became Nobel laureates as their mentor. Among Thomson’s prominent students were Ernest Rutherford, who discovered the atomic nucleus and Niels Bohr, who developed the revolutionary Bohr’s model of the atom. Based on the success of Thomson’s students, we can say that he was not only a talented scientist and physicist but also an outstanding teacher who instilled a love for exact sciences, passed on his experience and helped his students make breakthrough discoveries.
Joseph Thomson received many awards for his contribution to science and the development of scientific society. Perhaps, one of the most significant ones was the Nobel Prize in Physics, which he received in 1906. He was also knighted in 1908 and was awarded the Order of Merit in 1912. Later, in 1918, Thomson became Master of Trinity College, Cambridge. Throughout his life, Thomson remained devoted to his work. He continued to explore the mysteries of the physics world until his death in 1940. His ashes rest in Westminster Abbey, next to the famous mathematician, physicist, astronomer, alchemist, theologian Isaac Newton and his talented and no less outstanding student Ernest Rutherford.
The memory of the outstanding scientist lives on not only in his research and discoveries. In particular, in the 1990s, the Thomson (Th) was proposed as a unit of mass-to-charge ratio in mass spectrometry in memory of the outstanding Manchester scientist. In addition, there is a street on the territory of the University of Cambridge named in honour of the physicist, J J Thomson Avenue. The International Mass Spectrometry Foundation awards the Thomson Medal for outstanding scientific achievements, honouring the talented scientist.